
Aluminum Chloride Electron Dot Diagram For Aluminum Chloride
Lewis Dot Structure for Al2O3 (Aluminum Oxide) Geometry of Molecules 3.28K subscribers Subscribe Subscribed 11 Share 1.1K views 1 year ago Lewis Structure Hello there! Today in this video.
Dwayne's Education Blog Dwayne Schnell's ePortfolio
The aluminum oxide (Al 2 O 3) or alumina, which is largely applied in several arenas such as electronic, ceramics, high thermal material and catalysts etc.The current work was design with aluminum oxide quantum dots (γ-Al 2 O 3 QDs) and plays a crucial role for the concentration based analysis of hydrogen peroxide (H 2 O 2, referred to as HP).The γ-Al 2 O 3 was synthesized via hydrothermal.
14+ Cacl2 Lewis Structure Robhosking Diagram
This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

Explain The Formation Of Ionic Compound Al2o3 With Electron Dot Structure Chemistry Metals
Aluminum oxide (Al2O3) is a compound composed of two aluminum (Al) atoms and three oxygen (O) atoms. To determine the Lewis dot diagram for Al2O3, we need to know the number of valence electrons for each element. Aluminum has three valence electrons, while oxygen has six valence electrons.

Lewis Structure of Al2O3, Aluminum Oxide YouTube
Lewis Structure of Al2O3, Aluminum Oxide chemistNATE 259K subscribers Subscribe 47K views 2 years ago Lewis Structures TWO Aluminum atoms, metals with 3 electrons in its outer shell, requires.

How to form bond of al2o3 by electron dot structure? Brainly.in
Visit http://ilectureonline.com for more math and science lectures!In this video I will show the Lewis structure for ionic compound for aluminum oxide, Al2O3.

Electron Dot Diagram For Al
Learn the electron dot structure of Al2O3 in the easiest way. You can also watch the complete video on electron dot structure of other compounds herehttps://.

draw electron dot structure of aluminium oxide Brainly.in
The detailed geometric and electronic insights into the interface structure and evolution expand our understanding of this fundamental interface and have important implications for the engineering and design of Al /Al-based corrosion coatings with enhanced barrier properties, controllable transistor technologies, and noise-free superconducting q.

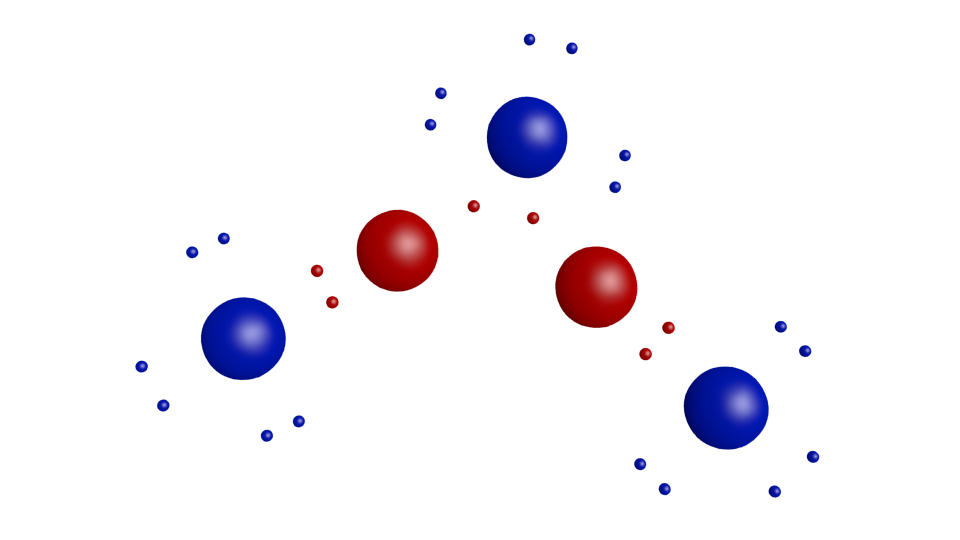
Different structures of (Al2O3)2 (Al and O atoms are depicted by red... Download Scientific
Aluminium oxide Structure (Al 2 O 3 Structure) Properties of Aluminium oxide - Al 2 O 3 Chemical Properties of Aluminium Oxide 1. Reaction with sodium hydroxide Aluminium oxide reacts with sodium hydroxide to produce sodium aluminate and water. This reaction takes place at a temperature of 900-1100°C.

Ionic Bonding Examples (1.3.5) CIE AS Chemistry Revision Notes 2022 Save My Exams
Science Chemistry Chemistry questions and answers Write the Lewis structure for Al_2O_3. Draw the Lewis dot structure for Al_2O_3. Include all lone pairs of electrons. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer

Aluminium oxide Al₂O₃ Molecular Geometry Hybridization Molecular Weight Molecular
A step-by-step explanation of how to draw the Al2O3 Lewis Dot Structure.For Al2O3 we have an ionic compound and we need to take that into account when we dra.

Is CS2 (Carbon disulfide) Ionic or Covalent/Molecular? YouTube
Lewis Structure of Al2O3, Aluminum Oxide Aluminium oxide is a solid ionic compound, made from atoms of one metal (Aluminum) that have lost three electrons each to become +3 cations, and atoms of a non-metal (oxygen) which have gained two electrons each to become -2 anions. The numbers 3 and 2 have a lowest common multiple of 6.

How to Draw the Lewis Dot Structure for Al2O3 Aluminum oxide YouTube
Calculate Lewis Dot Structure for Al2O3. Assign Electrons. In Al2O3 there are 5 atoms sharing 4 bonds for which we need to assign the electrons in order to find the oxidation numbers. Once you have drawn the lewis diagram for Al 2 O 3, you can look at each bond and assign its electrons to the more electronegative species.

A, 26, 380, (2008). Properties of Al2O3 Aluminum oxide can possess several different structures. As the compound is annealed for longer periods of time and at higher temperatures, it changes structure, which ultimately influences the properties of the compound. The transition of Al2O3 with heat:

explain the formation of ionic compound al2o3 with electron dot structure. (given. atomic no. of
It is the combination of metal and non-metal compounds. Thus it forms an ionic compound. Al2O3 is composed of 2 aluminum metals and 3 oxygen atoms. Both Al atoms lost their 3 valence electrons to 3 O atoms thus it has a +3 charge. The 3 O atoms gain 2 electrons from 2 Al atoms thus it has a -2 charge on it.

CLASS 9TH SCIENCE TABLE 3.6 POLYATOMIC ION AL2O3 LEWIS DOT STRUCTURE. YouTube
Amphoteric nature Aluminium oxide is an amphoteric substance, meaning it can react with both acids and bases, such as hydrofluoric acid and sodium hydroxide, acting as an acid with a base and a base with an acid, neutralising the other and producing a salt. Al 2 O 3 + 6 HF → 2 AlF 3 + 3 H 2 O